TreatMed’s on-site and off-site procedures, framework, responsibilities, policies,and practices regarding the safe management and disposal of Regulated Medical Waste (RMW) are in accordance with local, state, and federal laws and regulations including DOT and OSHA guidelines and standards.
Compliance

To ensure public safety and environmental protection, federal, state, and local laws require Regulated Medical Waste to be treated and rendered non-infectious before it can be disposed of as a solid municipal waste. It is unlawful to reduce, reuse, or recycle any infectious waste prior proper treatment and disposal. Healthcare providers, waste generators, and waste management companies are required to properly handle, treat, and dispose of the generated biomedical waste without endangering the public health and the environment.
The following regulatory authorities play a central role in regulating medical waste management and disposal:
- The State’s Department of Environmental or Health
- County and Local Authorities
- The Environmental Protection Agency (EPA)
- Department of Labor (DOL)
- - Occupational Safety and Health Administration (OSHA)
- Department of Transportation (DOT)
- Department of Health and Human Services (DHHS)
- - Centers for Disease Control and Prevention (CDC)

Our Sterilizers, the ECODAS SYSTEMS, are designed and manufactured in accordance with the following norms:
- EN ISO 9001:2008 – Quality Management Systems
- The International Certification Network Quality Management System
- The American Society of Mechanical Engineers (ASME)
- Canada Technical Safety Standards Association (TSSA)
- European Directive (CE) Quality Assurance System
- Japanese Ministry of Health, Labor and Welfare (MHLW)
- Gosstandart of Russia (GOST R)
- China Manufacture License of Special Equipment (MLSE)

The ECODAS process is also recognized and accepted worldwide by the Ministries of Health and Environment of numerous countries among them are: Argentina, Brazil, Cameron, China, Croatia, Cyprus, Denmark, Egypt, France, Ghana, Greece, Honduras, Hungary, India, Iran, Ivory-Cost, Jamaica, Japan, Jordan, Kuwait, Lebanon, Mexico, Morocco, Panama, Peru, Poland, Romania, Russia, Spain, Tunisia, Turkey, UAE, Ukraine, United Kingdom, Venezuela, etc.
The ECODAS SYSTEM has been accepted and approved in all 50 states as an alternative technology to treat Regulated Medical Waste.
The ECODAS system and its process were evaluated, tested, and approved by:
- WNWN (Waste Not, Want Not) International, Inc. a U.S. based company with over 20 years of experience in all facets of medical waste management at the domestic and international level.
- Pasteur Institute (ISO/IEC 17025 International Standard Accredited Laboratory – Accreditation No. 1-2215). The International Laboratory Accreditation Cooperation (ILAC) is an international cooperation of laboratory and inspection accreditation bodies established to promote the acceptance of technical test and calibration data worldwide. ILAC’s mission of “One test, one accreditation–accepted everywhere” is achieved through Mutual-Recognition Arrangements (MRAs) among the accreditation bodies themselves. The United States is a direct ILAC-MRA signatory. The U.S. Department of Commerce, National Institute of Standards and Technology, the U.S. Department of Defense, and the Nuclear Regulatory Commission use the ILAC MRA. Trade agreements now include references to the ILAC MRA.
The microbiological analysis and evaluation were conducted following the State and Territorial Association on Alternative Treatment Technologies (STAATT) testing protocol using bacterial spore strips (Stearothermophilus) and M. Terrae with different waste compositions. Samples were taken at critical points and at different times during treatment cycles. All test results demonstrated No growth of G. Stearothermophilus in any of the spores test samples indicating a >6 log10 reduction, and No growth of M. Terrae in any of the tissue samples indicating a > 6 log10 reduction of recoverable organisms. The Microbiological evaluation and test results demonstrate the ability of ECODAS Systems to effectively treat all types of regulated medical waste. Effluent water and air emissions were also tested and found to be acceptable and within the regulatory guidelines.
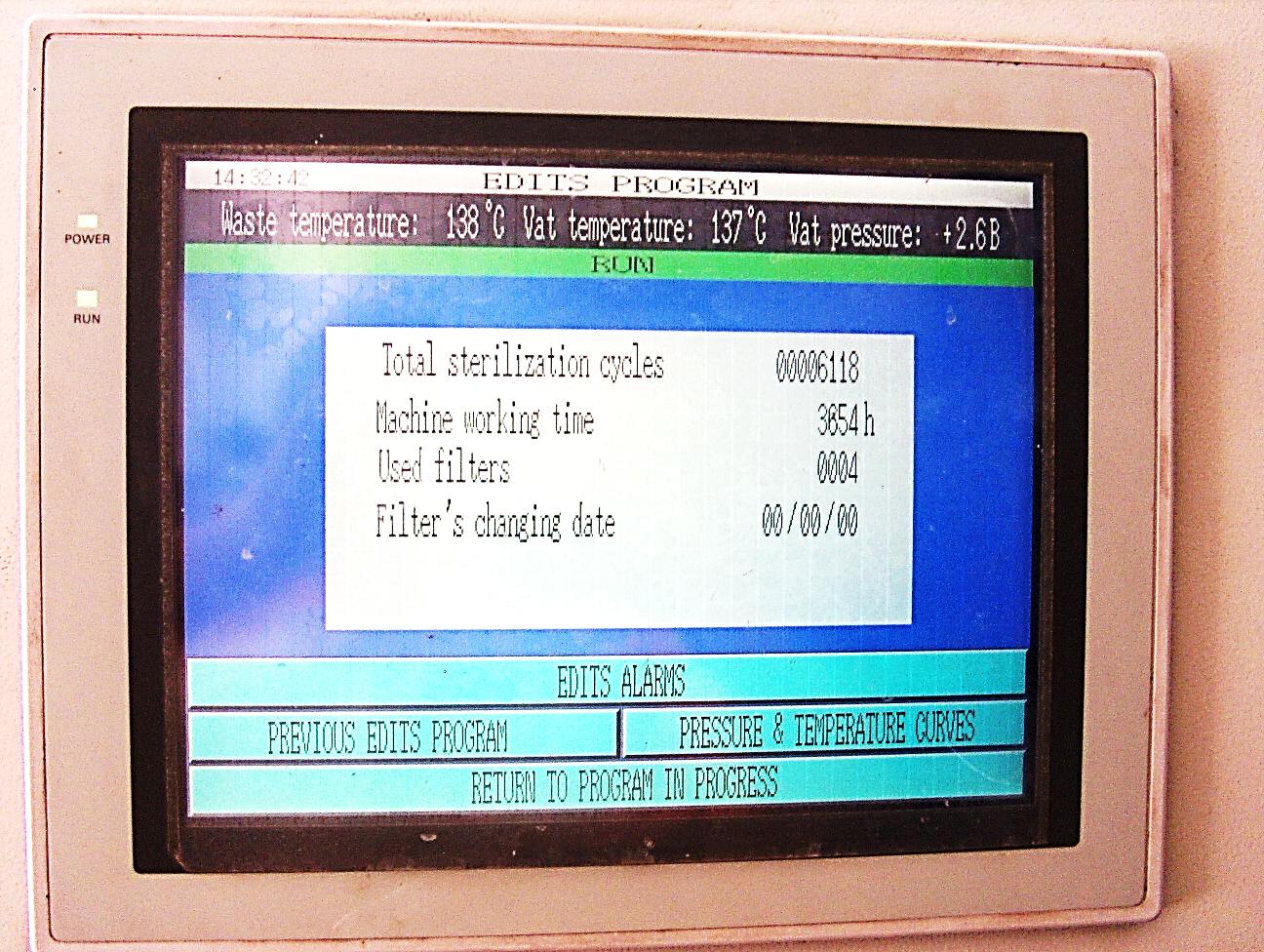
The whole operations and sequences of the ECODAS SYSTEM are entirely computerized and regularly controlled by an advanced programmable microprocessor offering reliable, simple, consistent, and safe treatment process. This Programmable Logic Controller (PLC) monitors, regulates, and maintains the operating parameters during the entire length of each automated treatment cycle.
Pneumatic, electrical, and mechanical systems are engaged, controlled, continuously monitored and maintained throughout the treatment cycle of the machine to ensure total control, safe operation, reliable run, and effective treatment process are achieved every time and all the time.

ECODAS has a reliable safety track record with NO environmental or industrial incident of any sort since introducing its first system in 1993. This dependable performance and consistent history testify to the level of expertise and social responsibility of this advanced green technology.
ECODAS clean autoclaving technology does not generate any pollutants, harmful or toxic materials because:
- New pollutants are not formed in the treatment process due to its lower operating temperatures compared to traditional burning technologies.
- No toxic or reactive chemicals are used in the treatment process.
Additionally, ECODAS design includes programmable logic, safety mechanism, and management process flow through tanks, pumps, and valves enabling a control of all air, steam, and water flow without having any impact on the environment.
